Mobility is complicated:
And yet mobility drives spatio-temporal spread:
Pathogens travel along trade routes
In ancient times, trade routes were relatively easy to comprehend
With acceleration and globalization of mobility, things change
All migrants/travellers carry with them their "health history"
Pathogens ignore borders and politics
Index case for international spread arrives HKG 21 February 2003
Last country with local transmission (Taiwan) removed from list 5 July 2003
8273 cases in 28 countries
(Of these cases, 1706 were HCW)
775 deaths (CFR 9.4%)
Assume no birth or death. Balance inflow and outflow
when we write
with incidence
with incidence

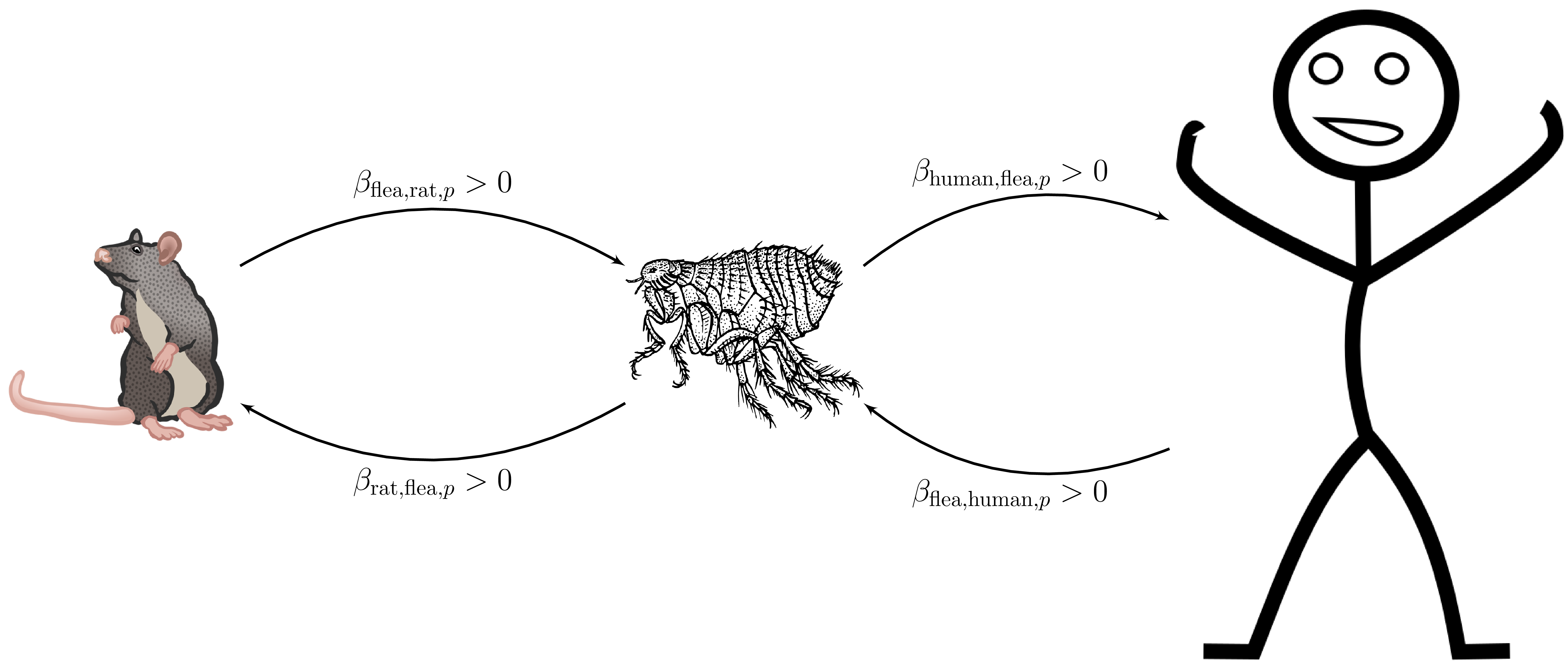

with incidence
For
where
For simplicity, consider
with incidence
System of
System of
However, a lot of structure:
Consider behaviour of
So
We have
Write this in vector form
where
Consider a compartment
Recall that
Suppose movement rates equal for all compartments, i.e.,
Then
Equilibria
given, of course, that
Using a spectrum shift,
This gives a constraint: for total population to behave well (in general, we want this), we must assume all death rates are positive
Assume they are (in other words, assume
Indeed, we have
Since we now assume that
Assume
From Foster and Jacquez, Multiple zeros for eigenvalues and the multiplicity of traps of a linear compartmental system, Mathematical Biosciences (1975)
Recall that
Suppose movement rates similar for all compartments, i.e., the zero/nonzero patterns in all matrices are the same but not the entries
Let
and
Then we have
Me, roughly every 6 months: Oooh, coooool, a linear differential inclusion!
Me, roughly 10 minutes after that previous statement: Quel con!
Indeed
So no luck there..
However, non lasciate ogne speranza, we can still do stuff!
Assume system at equilibrium and
Want to solve for
where
Recall second equation:
So unique solution
Is it?
We have been here before!
From spectrum shift,
So, given
DFE has
Recall:
Second point
So DFE makes sense with
Use next generation method with
Differentiate w.r.t.
Note that
whenever
If
If
In both cases,
where
where
Next generation matrix
where
i.e.,
Define
Then the DFE
is locally asymptotically stable if
From PvdD & Watmough, Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission, Bulletin of Mathematical Biology 180(1-2): 29-48 (2002)
JA & S Portet. Epidemiological implications of mobility between a large urban centre and smaller satellite cities. Journal of Mathematical Biology 71(5):1243-1265 (2015)
Winnipeg as urban centre and 3 smaller satellite cities: Portage la Prairie, Selkirk and Steinbach
| City | Pop. (2014) | Pop. (now) | Dist. | Avg. trips/day |
|---|---|---|---|---|
| Winnipeg (W) | 663,617 | 749,607 | - | - |
| Portage la Prairie (1) | 12,996 | 13,270 | 88 | 4,115 |
| Selkirk (2) | 9,834 | 10,504 | 34 | 7,983 |
| Steinbach (3) | 13,524 | 17,806 | 66 | 7,505 |
Assume
Now,
Computed for all pairs
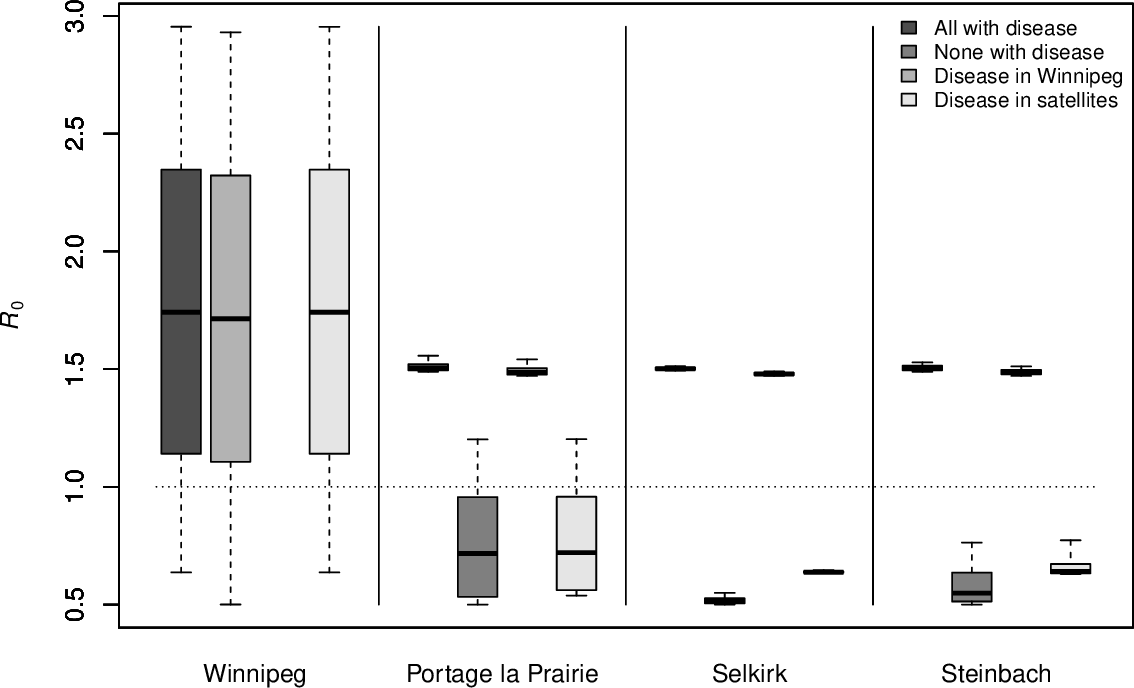
with disease:
PLP and Steinbach have comparable populations but with parameters used, only PLP can cause the general
This is due to the movement rate: if
since
Movement rates to and from PLP are lower
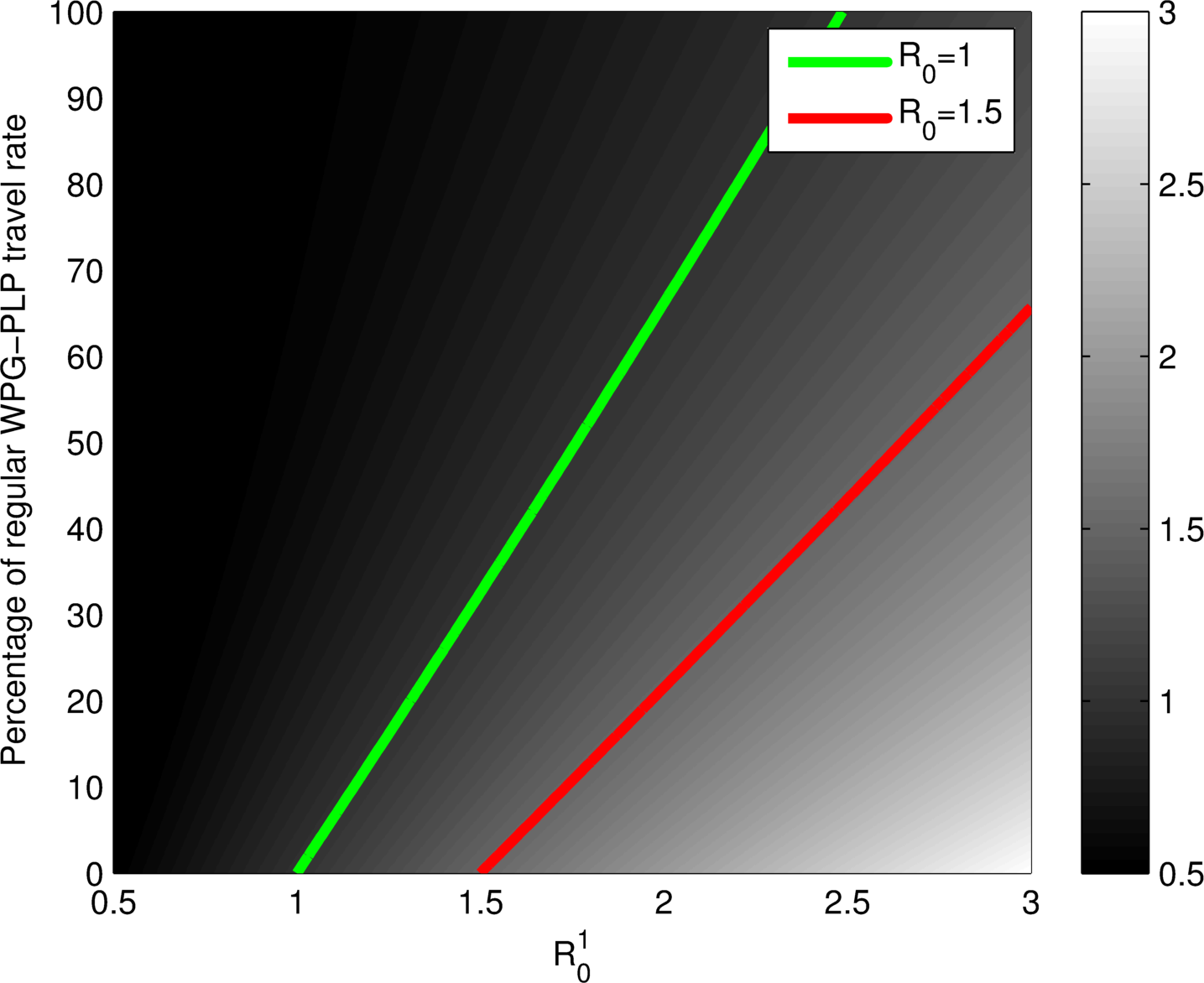
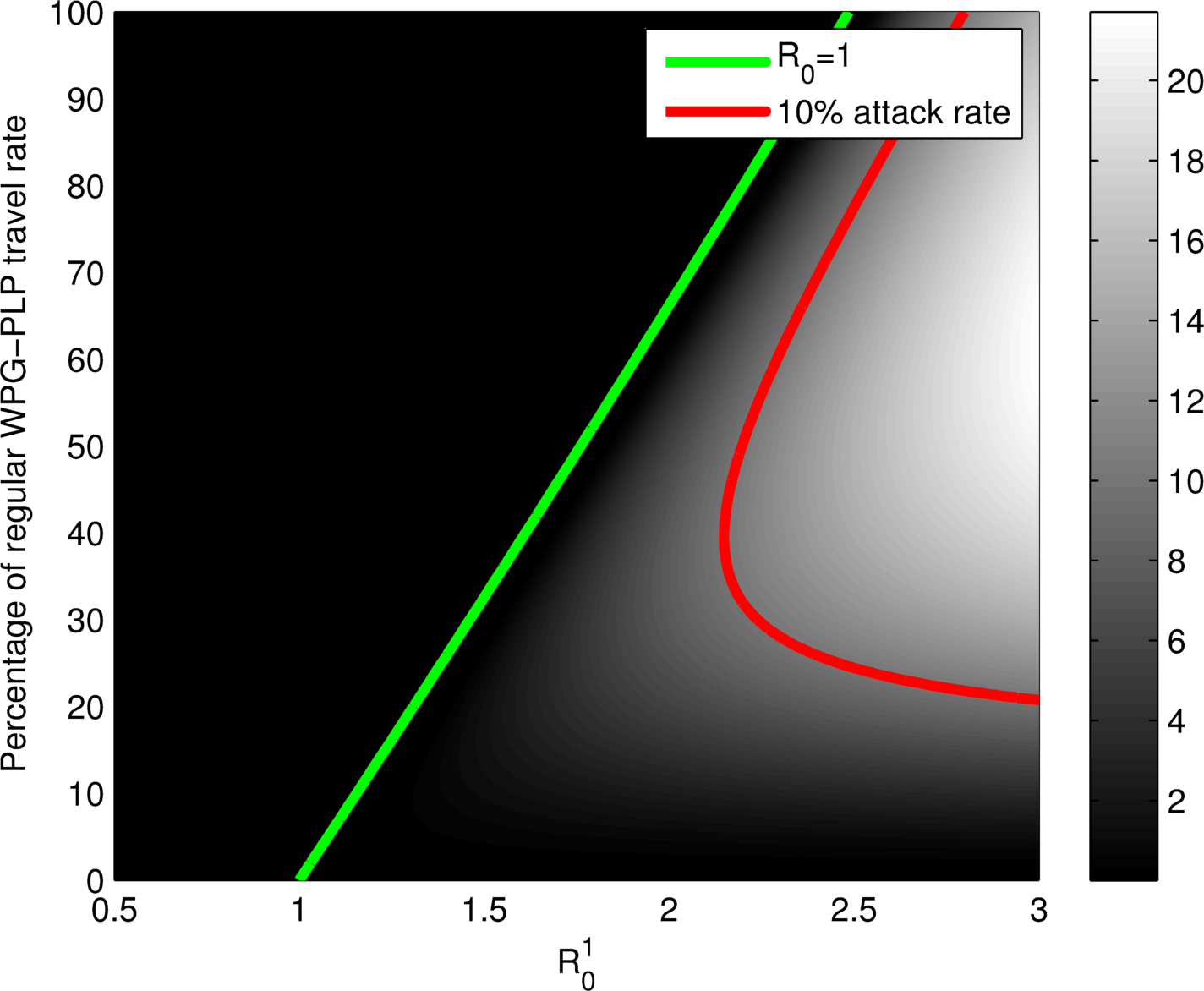
Plots as functions of
Given
with known properties, what is known of
YES, coupling together backward bifurcating units can lead to a system-level backward bifurcation
JA, Ducrot & Zongo. A metapopulation model for malaria with transmission-blocking partial immunity in hosts. Journal of Mathematical Biology 64(3):423-448 (2012)
"Converse" problem to inheritance problem. Given
with known properties, does
exhibit some behaviours not observed in the uncoupled system?
E.g.: units have
Can there be situations where some patches are at the DFE and others at an EEP?
This is the problem of mixed equilibria
This is a metapopulation-specific problem, not one of inheritance of dynamical properties!
[Patch level] Patch
[Metapopulation level] A population-free equilibrium has all patches empty. A metapopulation disease-free equilibrium has all patches at the disease-free equilibrium for the same compartments. A metapopulation endemic equilibrium has all patches at an endemic equilibrium
A mixed equilibrium is an equilibrium such that
E.g.,
is mixed, so is
Suppose that movement is similar for all compartments (MSAC) and that the system is at equilibrium
Note that MSAC
More is needed on inheritance problem, in particular GAS part (Li, Shuai, Kamgang, Sallet, and older stuff: Michel & Miller, Šiljak)
Incorporate travel time (delay) and events (infection, recovery, death ..) during travel
What is the minimum complexity of the movement functions
required to observe a metapopulation-induced behaviour?
pop = c(34.017, 1348.932, 1224.614, 173.593, 93.261) * 1e+06
countries = c("Canada", "China", "India", "Pakistan", "Philippines")
T = matrix(data =
c(0, 1268, 900, 489, 200,
1274, 0, 678, 859, 150,
985, 703, 0, 148, 58,
515, 893, 144, 0, 9,
209, 174, 90, 2, 0),
nrow = 5, ncol = 5, byrow = TRUE)
p = list()
# Use the approximation explained in Arino & Portet (JMB 2015)
p$M = mat.or.vec(nr = dim(T)[1], nc = dim(T)[2])
for (from in 1:5) {
for (to in 1:5) {
p$M[to, from] = -log(1 - T[from, to]/pop[from])
}
p$M[from, from] = 0
}
p$M = p$M - diag(colSums(p$M))
p$P = dim(p$M)[1]
p$eta = rep(0.3, p$P)
p$epsilon = rep((1/1.5), p$P)
p$pi = rep(0.7, p$P)
p$gammaI = rep((1/5), p$P)
p$gammaA = rep((1/3), p$P)
# The desired values for R_0
R_0 = rep(1.5, p$P)
Save index of state variable types in state variables vector (we have to use a vector and thus, for instance, the name "S" needs to be defined)
p$idx_S = 1:p$P
p$idx_L = (p$P+1):(2*p$P)
p$idx_I = (2*p$P+1):(3*p$P)
p$idx_A = (3*p$P+1):(4*p$P)
p$idx_R = (4*p$P+1):(5*p$P)
# Set initial conditions. For example, we start with 2
# infectious individuals in Canada.
L0 = mat.or.vec(p$P, 1)
I0 = mat.or.vec(p$P, 1)
A0 = mat.or.vec(p$P, 1)
R0 = mat.or.vec(p$P, 1)
I0[1] = 2
S0 = pop - (L0 + I0 + A0 + R0)
# Vector of initial conditions to be passed to ODE solver.
IC = c(S = S0, L = L0, I = I0, A = A0, R = R0)
# Time span of the simulation (5 years here)
tspan = seq(from = 0, to = 5 * 365.25, by = 0.1)
Let us take
for (i in 1:p$P) {
p$beta[i] =
R_0[i] / S0[i] * 1/((1 - p$pi[i])/p$gammaI[i] + p$pi[i] * p$eta[i]/p$gammaA[i])
}
SLIAR_metapop_rhs <- function(t, x, p) {
with(as.list(p), {
S = x[idx_S]
L = x[idx_L]
I = x[idx_I]
A = x[idx_A]
R = x[idx_R]
N = S + L + I + A + R
Phi = beta * S * (I + eta * A) / N
dS = - Phi + MS %*% S
dL = Phi - epsilon * L + p$ML %*% L
dI = (1 - pi) * epsilon * L - gammaI * I + MI %*% I
dA = pi * epsilon * L - gammaA * A + MA %*% A
dR = gammaI * I + gammaA * A + MR %*% R
dx = list(c(dS, dL, dI, dA, dR))
return(dx)
})
}
# Call the ODE solver
sol <- ode(y = IC,
times = tspan,
func = SLIAR_metapop_rhs,
parms = p,
method = "ode45")
Suppose demographic EP is
Want to maintain
So take
Then
and thus if
Easy to think of situations where the diagonal will be dominated by the off-diagonal, so
Space is a fundamental component of the epidemic spread process and cannot be ignored, both in modelling and in public health decision making
One way to model space is to use metapopulation models
Metapopulation models are easy to analyse locally, give interesting problems at the global level
Simulation (deterministic and stochastic) can be costly in RAM and cycles but is easy
Metapopulation models are not the only solution! (see network models, individual-based models, ..)
<div style = "text-align: justify">
</div>
<p style="margin-bottom:-1.5cm;"></p>